Abstract
Background: Crenolanib is a type I oral pan-FLT3 inhibitor with high potency and selectivity against FLT3-ITD and FLT3-tyrosine kinase domain (TKD) mutations. Crenolanib has demonstrated a clinical activity as a single agent in heavily treated acute myeloid leukemia (AML) patients (pts). Preclinical studies have shown an antileukemic synergistic effect of the combination of crenolanib with cytotoxic agents. We report here the final analysis of an open label, dose escalation, two-arm, phase I/II trial of crenolanib combined with standard chemotherapy in pts with relapsed/refractory (R/R) FLT3 mutant AML.
Methods: Adult pts with a diagnosis of R/R FLT3 mutant AML were enrolled. Pts were assigned per physician's choice to either crenolanib in combination with higher intensity salvage chemotherapy (Arm1) or crenolanib with 5-azacitidine (Arm2). Higher intensity chemotherapy options consisted of either IA (Idarubicin (Ida) 12 mg/m2 for 3 days (d) with cytarabine (AraC) 1.5 g/m2 for 4 d (3 d if age > 60 yrs)). On a later amendment two other options were added: FLAG-Ida (Fludarabine 30 mg/m2, AraC 2g/m2 each for 5 d, and Ida 8 mg/m2 for 3 d), or MEC (Mitoxantrone 8 mg/m2, etoposide 100 mg/m2, AraC 1g/m2 all for 5 d). 5-azacitidine was given at 75 mg/m2/d for 7d each cycle. Standard rolling-6 design was implemented with dose escalation of crenolanib as follows: 60 mg TID (dose level 1), 80 mg TID (dose level 2), and 100 mg TID (dose level 3). Crenolanib was given continuously starting at the end of chemotherapy, and discontinued 3d before the next cycle. Responding pts were eligible to proceed to allogeneic hematopoietic cell transplant (alloHCT) or consolidation with AraC (750 mg/m2 for 3d) and Ida (8 mg/m2 for 2d) followed by crenolanib up to 6 cycles. Pts could continue on maintenance with single-agent crenolanib up to 1 year. Pts on Arm2 could continue combination therapy until progression or unacceptable toxicity. The primary objective was to determine the dose limiting toxicity (DLT) and maximal tolerated dose of crenolanib-based combinations, as well as overall response rates (ORR), including complete remission (CR), CR with incomplete blood count recovery (CRi), and partial remission (PR). Secondary objectives are duration of response, relapse free survival, and overall survival.
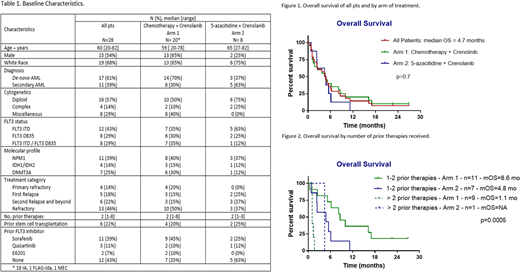
Results: Total 28 pts were treated. Baseline characteristics are summarized in Table 1. All 3 dose escalation cohorts have been completed. 20 pts received crenolanib in combination with salvage chemotherapy, and 8 with 5-azacitidine. 16 (57%) pts had received prior FLT3 inhibitors including sorafenib (n=11), quizartinib (n=3), E6201 (n=2). The median number of cycles received was 1 (range, 1-14). No DLTs were observed at any of the dose levels explored. Non-hematologic adverse events possibly related to crenolanib were all grade 1 or 2 in severity, including nausea (n=1), vomiting (n=2), fatigue (n=2), diarrhea (n=1), abdominal pain (n=1), muscular weakness (n=1), hypotension (n=1). No deaths were attributed to crenolanib. The ORR in 24 pts evaluable for response was 11 (46%) including CR (n=3), CRi (n=7), and PR (n=1). Three (11%) pts had hematologic improvement with bone marrow blast count reduction of at least 50%. Four pts were not evaluable for response due to early death3 from infection, 1 stopped therapy early for unrelated reasons). The median time to response was 29 days (range, 19-116). Among responders, 4 (36%) pts achieved negative minimal residual disease by flow cytometry after a median of 3.2 mo (range, 0.7-3.7). Five pts received consolidation with alloHCT, and 2 other pts had alloHCT after subsequent salvage therapy. One pt received crenolanib maintenance after transplant. The median OS (mOS) was 4.7 (0.4-27) mo (Figure 1); median RFS was 4 (1-23) mo. Of 18 pts who received one or 2 prior therapies, 9 (50%) pts achieved CR/CRi (including 3 of 9 pts with prior exposure to FLT3 inhibitors) and 5 (28%) received subsequent alloHCT. The mOS for pts who received ≤ 2 prior therapies was 6.2 mo versus 1.5 mo for pts who received ≥ 3 prior therapies (p=0.0002). OS by treatment arm and prior therapies is shown in Figure 2.
Conclusion: Full dose crenolanib (100 mg TID) can be safely combined with chemotherapy in R/R FLT3 mutant AML. ORR can reach up to 50% with the combination, even with prior exposure to FLT3 inhibitors, and particularly among Arm 1 pts with ≤ 2 prior therapies (mOS=8.6 mo). The study was terminated at the sponsor's request.
Jabbour:Takeda: Consultancy, Research Funding; Novartis: Research Funding; Pfizer: Consultancy, Research Funding; Abbvie: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding. Wierda:Genentech: Research Funding; AbbVie, Inc: Research Funding. Daver:ImmunoGen: Consultancy; Alexion: Consultancy; Pfizer: Research Funding; Otsuka: Consultancy; Novartis: Consultancy; Sunesis: Research Funding; Karyopharm: Consultancy; Pfizer: Consultancy; Kiromic: Research Funding; BMS: Research Funding; ARIAD: Research Funding; Sunesis: Consultancy; Daiichi-Sankyo: Research Funding; Novartis: Research Funding; Incyte: Research Funding; Karyopharm: Research Funding; Incyte: Consultancy. Konopleva:Stemline Therapeutics: Research Funding. Andreeff:SentiBio: Equity Ownership; Aptose: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Oncolyze: Equity Ownership; Daiichi-Sankyo: Consultancy, Patents & Royalties: MDM2 inhibitor activity patent, Research Funding; Celgene: Consultancy; Oncoceutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Research Funding; Amgen: Consultancy, Research Funding; Eutropics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Consultancy; United Therapeutics: Patents & Royalties: GD2 inhibition in breast cancer ; Reata: Equity Ownership. Pemmaraju:cellectis: Research Funding; samus: Research Funding; SagerStrong Foundation: Research Funding; abbvie: Research Funding; celgene: Consultancy, Honoraria; stemline: Consultancy, Honoraria, Research Funding; Affymetrix: Research Funding; novartis: Research Funding; plexxikon: Research Funding; daiichi sankyo: Research Funding. Kadia:Novartis: Consultancy; Abbvie: Consultancy; Celgene: Research Funding; Jazz: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Takeda: Consultancy; Jazz: Consultancy, Research Funding; BMS: Research Funding; Amgen: Consultancy, Research Funding; Celgene: Research Funding; Novartis: Consultancy; BMS: Research Funding; Pfizer: Consultancy, Research Funding; Abbvie: Consultancy; Takeda: Consultancy; Amgen: Consultancy, Research Funding. Ravandi:Jazz: Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Jazz: Honoraria; Orsenix: Honoraria; Sunesis: Honoraria; Xencor: Research Funding; Bristol-Myers Squibb: Research Funding; Abbvie: Research Funding; Sunesis: Honoraria; Orsenix: Honoraria; Macrogenix: Honoraria, Research Funding; Seattle Genetics: Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria; Abbvie: Research Funding; Macrogenix: Honoraria, Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Seattle Genetics: Research Funding; Bristol-Myers Squibb: Research Funding; Xencor: Research Funding. Cortes:Pfizer: Consultancy, Research Funding; Astellas Pharma: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Arog: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal